usp class vi pdf
The USP Class VI compounds must be. To confirm accuracy and reproducibility USP Reference Standards are rigorously tested and evaluated by multiple independent laboratories including USP commercial regulatory and.
![]()
Usp Class Vi Silicone Is Independently Certified For Biocompatibility Specialty Silicone Products Inc
E6A53 A representative article comprised of two halves of E553 and a splice utilizing E6A53 has been.
. USP Class VI Certificate of Compliance Silicone Compound. There are six classes VI being the most rigorous. Interim Revision Announcement 2 87 Biological Reactivity Tests In Vitro Official November 1 2015 NaCl or serum-free mammalian cell culture media as Ex-Table 1.
A new USP Class VI-compliant substrate for manufacturing disposable microfluidic devices. USP Class VI plastic. To test medical device biocompatibility manufacturers often use USP procedures such as the USP In Vivo Biological Reactivity Tests Class I-VI Plastics Tests.
Class VI testing is aimed to. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. Parker Hannifin Corporation Engineered Polymer Systems Division 2220 South 3600 West Salt Lake City UT 84119 phone 801 972.
Results of tests are stated in the following Test Reports. Parker Hannifin Corporation Engineered Polymer Systems Division 2220 South 3600 West Salt Lake City UT 84119 phone 801 972. The Class Plastics tests.
USP USP class VI was especially devel-oped for the pharmaceutical industry. Used to classify plastics in Classes I - VI based on end use type and time of exposure of human tissue to plastics of which Class VI requires the most stringent testing of all the six classes. USP Class VI Certified Materials Contact Information.
Biocompatibility is historically referring to the USP class VI United States Pharmacopoeia for testing where class VI represents the highest class. Intracutaneous Test are used for elastomeric materials espe-1 USP High-Density Polyethylene RS. Guidelines of USP Class VI pharmaceutical approval.
Cially to elastomeric closures for which the appropriate Bio-Table 1. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. This information has been carefully prepared to help in selecting the cor-rect elastomer or.
USP Class VI Tests. USP Class IV USP Class V USP Class VI USP Class IV USP Class VI USP Class VI USP Class IV USP Class VI USP Class VI. SIMONA PP-H USP Class VI sheet material is easy to.
S-2013-01361SAMi released 26th July 2013 for - Biological reactivity test in vivo - Systemic. USP Class VI Certified Materials Contact Information. Lab on a Chip 2009.
Pharmacopeial Conven-tion USP is a non-commercial organisation that develops the standards for the quality of. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds.

Sanitary Gasket Material Guidelines Selection Rubber Fab

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

What Is Usp Class Vi Testing Tbl Plastics

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Aktaprocess Ge Healthcare Life Sciences Pdf Catalogs Technical Documentation
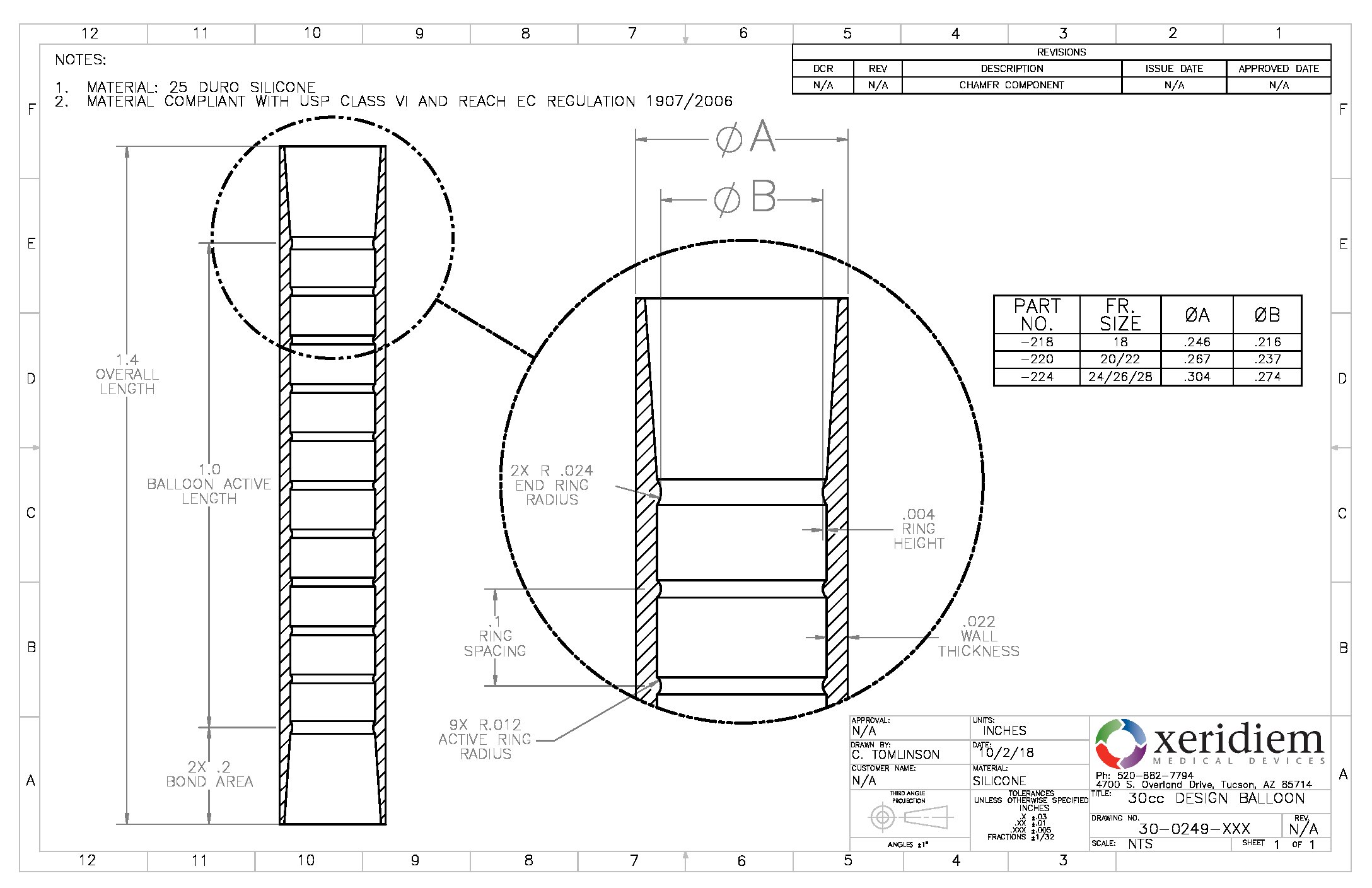
Balloon Silicone 6 8mm Od Spherical Quantity 10 Bag

Formlabs Biomed Clear Resin Cartridge 1l Dynamism
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
![]()
Ssp 2390 Series Specialty Silicone Products Inc

Usp Class Vi Testing Aft Fluorotec

Data Sheets Panacol Elosol Gmbh

Usp Y W Technologies Finish With The Best
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants